Biotechnology
Germ Control Protocol©
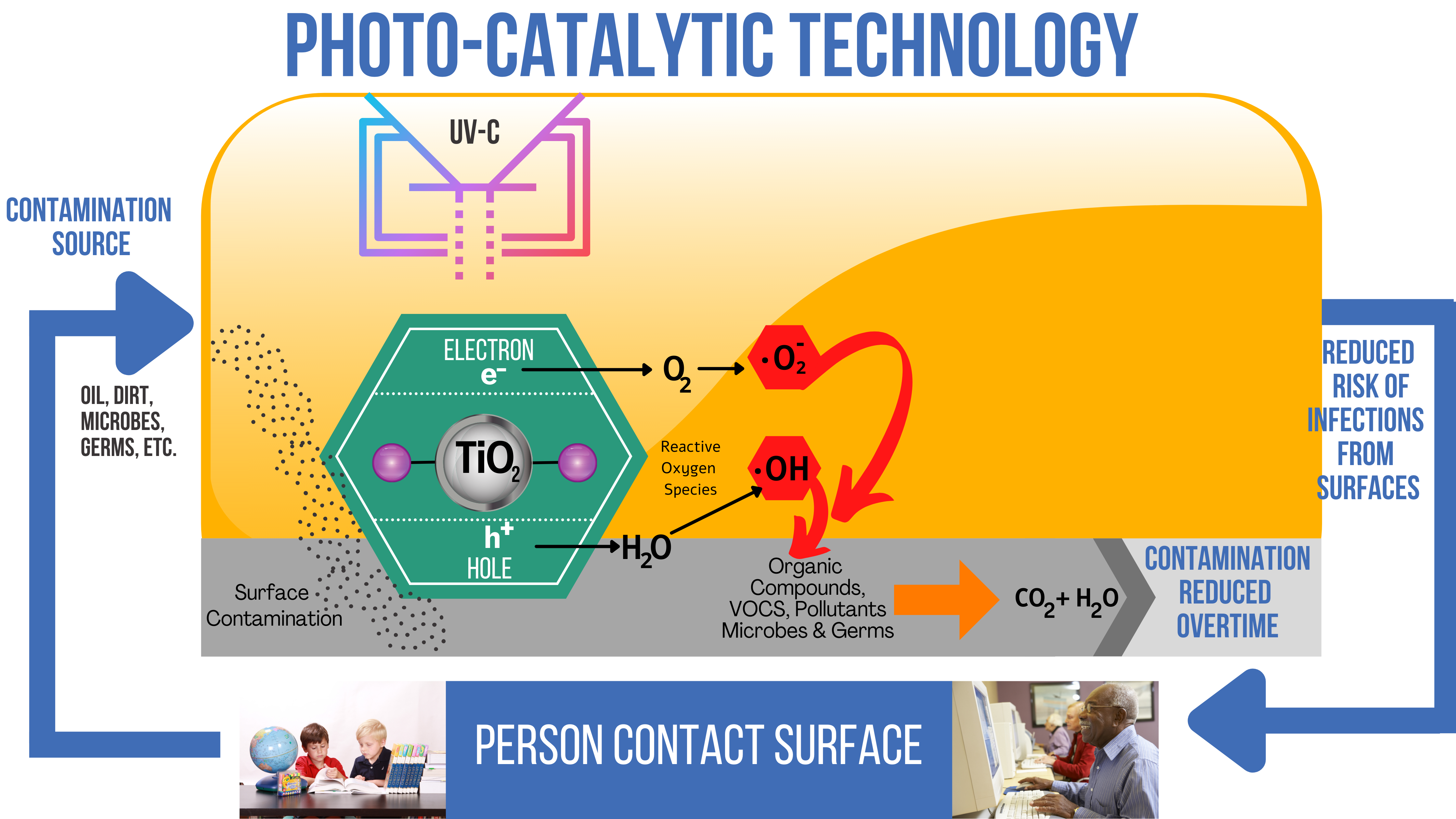
Our Germ Control Protocol© (GCP©) breaks down harmful microorganisms on surfaces for up to 12 months by combining two methods, photocatalysis and nanotechnology, into one, creating the most advanced light-activated surface coating on the market.
GCP© sprays a solution of titanium dioxide onto different surfaces, creating a “nanocoating” that catalyzes a reaction when exposed to light, resulting in hydroxyl radicals. These highly reactive radicals decompose and destroy cell membranes on contact.
The Germ Control Protocol© uses 254nm medical-grade Ultraviolet C (UV-C) lamps’ articulated arms to direct light beams directly onto surfaces treated with our solution of TiO₂, allowing for a faster photocatalytic oxidative reaction and resulting in a dual and prolonged germicidal action.
How Can gcp© guarantee 12 months?
Using the principle of photocatalytic oxidation, GCP© guarantees up to 12 months of protection against germs on surfaces
Our GCP© is a giant leap in the development of an effective weapon against harmful pathogens. Easy-to-clean surface nano-coatings are now standard in higher standards of germ protection. Nano-coating technologies will change traditional disinfection approaches and prevent transmission of infectious disease
A flat, smooth surface isn’t what it seems to be to the naked eye. When these surfaces are examined through a microscope, those seemingly flat and smooth surfaces are, in fact, micron- and nano-sized crevices and valleys where dust, bacteria, viruses, and other undesirable organic debris hides. Even water, the universal cleaning element, can only remove large dust particles by itself, leaving behind bacteria, viruses, and superfine contaminating particles.
GCP© employs a patented, easy-to-clean nanostructured coating that changes the hydrophobic properties of standard surfaces to hydrophilic, allowing water to penetrate deeper and wash away a larger portion of contaminants hidden on furniture doorknobs, handrails, and more.
Our nano-coating guarantees extended protection against harmful germs. GCP© combines the germicidal properties of two different agents for immediate sterilization that lasts, keeping surfaces protected for up to 12 months after the initial pathogen removal procedure.
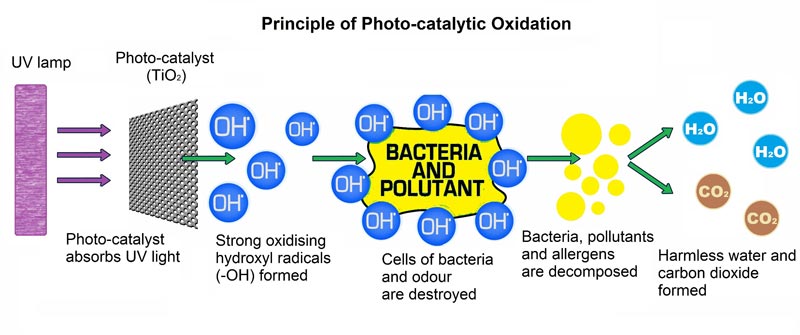
Photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate.
Photo-generated catalysis depends on the ability of the catalyst to create electron-hole pairs, which create free radicals (hydroxyl radical: •OH) able to undergo secondary reactions.
Photocatalyst has the following advantages over any current purification technologies:
- The real destruction of pollutants rather than a simple transfer on a substrate.
- Degradation of pollutants at ambient temperature and pressure.
- Adapted for an extensive range of pollutants (VOC, bacteria, mold).
- Long-lasting protection from germs on the surface.
Titanium Dioxide, also known as titanium (IV) oxide or titania, is the naturally occurring titanium oxide, chemical formula TiO2. Approved by the United States Food and Drug Administration (FDA) food testing laboratory, Titanium Dioxide is considered a safe substance and harmless to humans. It is commonly used in paint, printing ink, plastics, paper, synthetic fibers, rubber, condensers, painting colors and crayons, ceramics, electronic components, along with food and cosmetics. Many studies have been published using Titanium Dioxide as a Powerful Photocatalyst for the decomposition of organic compounds.
Titanium Dioxide molecules contain electrons that are confined to relatively narrow energy bands. The band of the highest energy-containing electrons is the valence band, while the band lying above the valence band, i.e., the conduction band, has very few electrons. The difference in energies between the valence band’s highest energy and the conduction band’s lowest energy is termed the bandgap energy. When a semiconductor absorbs a photon of energy equal to or greater than its bandgap, an electron may be promoted from the valence band to the conduction band leaving behind an electron vacancy or “hole” in the valence band. If charge separation is maintained, the electron and the hole may migrate to the catalyst surface, where they participate in a redox reaction with sorbed species.
When Titanium Dioxide exposes to light in the presence of water vapor, two highly reactive substances form hydroxyl radicals [OH] and a superoxide ion [O2-1]. It allows airborne VOCs’ oxidation into carbon dioxide and water at room temperature with UV or near- UV light source. It does not need different energy and uses clean energy found in ordinary life. Specific titanium dioxide has a powerful photocatalytic reaction. It has strong oxidation and decomposition strength.
The FDA food testing laboratory approved the use of Titanium Dioxide (TiO2 ) as a safe substance and harmless to humans. Our agent containing Titanium Dioxide complies with EPA regulations and CDC Guidelines for Disinfection and Sterilization in Healthcare Facilities.
In order to hold to the GCP©’s 12months guarantee, the surfaces must be deeply cleaned before applying the treatment, following the CDC guidelines: “If surfaces are dirty, clean them. Use detergent or soap and water prior to disinfection.”

- Hydroxyl radicals are among the strongest oxidizing species, even more potent than chlorine, ozone, and peroxide. They act as potent disinfecting agents by oxidizing microorganisms’ cells, rupturing them and causing vital composition leakage. Although not all hydroxyls are the same, hydroxyl radicals’ oxidation power has great germ-fighting benefits.
- Titanium Dioxide (TiO₂) is non-toxic and harmless to humans and animals. The photocatalytic reactions on which GCP© is based ensure long-lasting protection against germs for up to 12 months. Do not consume this chemical compound.
Titanium dioxide has strong oxidation effects on single-celled organisms, including all bacteria and fungus. This extreme oxidizing power can destroy bacteria’s cell membranes, causing cytoplasm leakage, and inhibiting its activity, resulting in its decomposition and death. Titanium oxide disinfection is three times stronger than chlorination and 1.5 times stronger than ozonation.
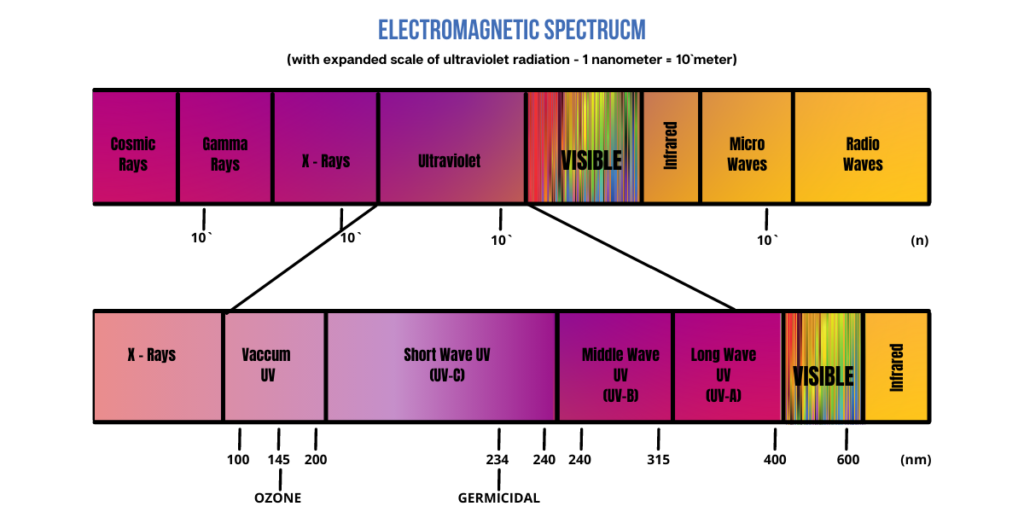
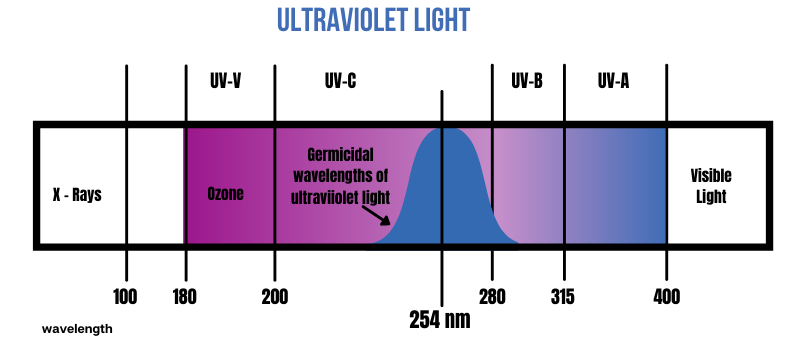
ULTRAVIOLET LIGHT
UV is generally divided into three sub-bands of light: UV-A, UV-B, and UV-C, ranging between 180 nm to 400 nm.
- UV-A has a longer wavelength and is not stopped by ozone. It can penetrate the middle layer of your skin and is associated with aging.
- UV-B rays have a short wavelength that reach the outer layer of your skin. They penetrate the ozone layer in an attenuated form and reaches the surface of the planet. It is used in medicine to treat certain diseases, however, it can cause sunburn, darkening and thickening of the skin’s outer layer, and various forms of skin cancer.
- UV-C has the shortest wavelength and is only exposed to humans through artificial sources, such as a lamp or laser, and can cause skin and eye burns. It is used to sanitize surfaces, water, and the air.
- UV-C radiation is the highest energy portion of the UV radiation spectrum, extending from about 180 to 280 nm.
- UV-C radiation is a known disinfectant for air, water, and nonporous surfaces.
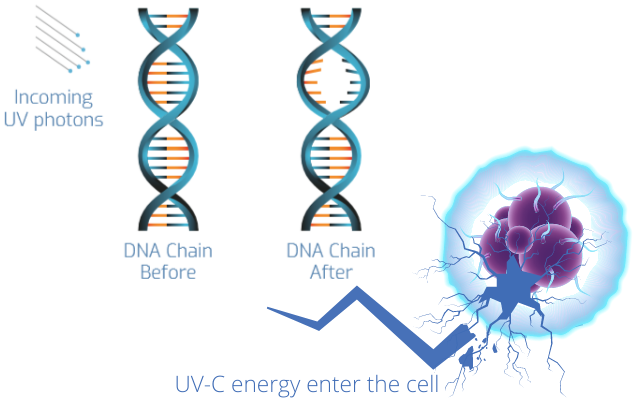
- UV-C radiation causes damage to microorganisms’ genomes, breaking bonds and forming lesions in nucleic acids, DNA, and RNA.
- Direct UV-C radiation to pathogens prevents both transcription and replication of the cellular or viral components, causing a germicidal region and ultimately lead to inactivation of the microorganisms.
- The UV-C method of pathogen inactivation has been in healthcare since 1930; in 2009, this technology started to be used to disinfect hospital surfaces.
- The use of UVGI does not change the cleaning protocols and it is safe only in the hands of a skilled and knowledgeable specialist, as Germ Control Technicians are.
- TiO2 with UV-C radiation creates an additional coating for:
- Patient rooms
- Operating Rooms (OR).
- Bathrooms.
- Emergency Rooms (ER).
- The out of patient care.
- Hospital beds
- Ambulances.
- Intensive Care Units (ICU)
- Other hospital areas.
As reported by Lindsay Kalter in WebMD Health News, “The sanitizing effects of UV lights have been seen with other coronaviruses, including the one that causes severe acute respiratory syndrome (SARS). Studies have shown that it can be used against other coronaviruses. One study found at least 15 minutes of UV-C exposure inactivated SARS, making it impossible for the virus to replicate.”
https://www.webmd.com/lung/news/20200519/coronavirus-puts-uv-in-the-disinfectant-spotlight
Pathogen decrease is related to the dose of the UV-C, depending on the equipment characteristics and application.
- The application’s angle on the disinfection area target.
- Power radiated by the device.
- Distance between the device and the surface to be disinfected.
- Surface exposure time.
GCP© uses lamps that have controls and quality assurance procedures to diminish the risk of human exposure to UV-C radiation, such as:
- A timer that delays the radiation exposure and gives the technician time to leave the area to be treated.
- A movement sensor that automatically turns off the device if a door opens or a person tries to get to the treated room without authorization.
- The GCP© determines the UV-C light time, combining the digital dose calculator’s usage and the square feet of an area, guaranteeing accurate radiation time to ensure a complete photocatalytic effect.
- The UV-C’s effects are not visible. Equipment operators measure the dose level applied with UV-C dosimeters photochromic measure cards.